Tech Topic | October 2020 Hearing Review
By Rachel Sharnetzka, AuD
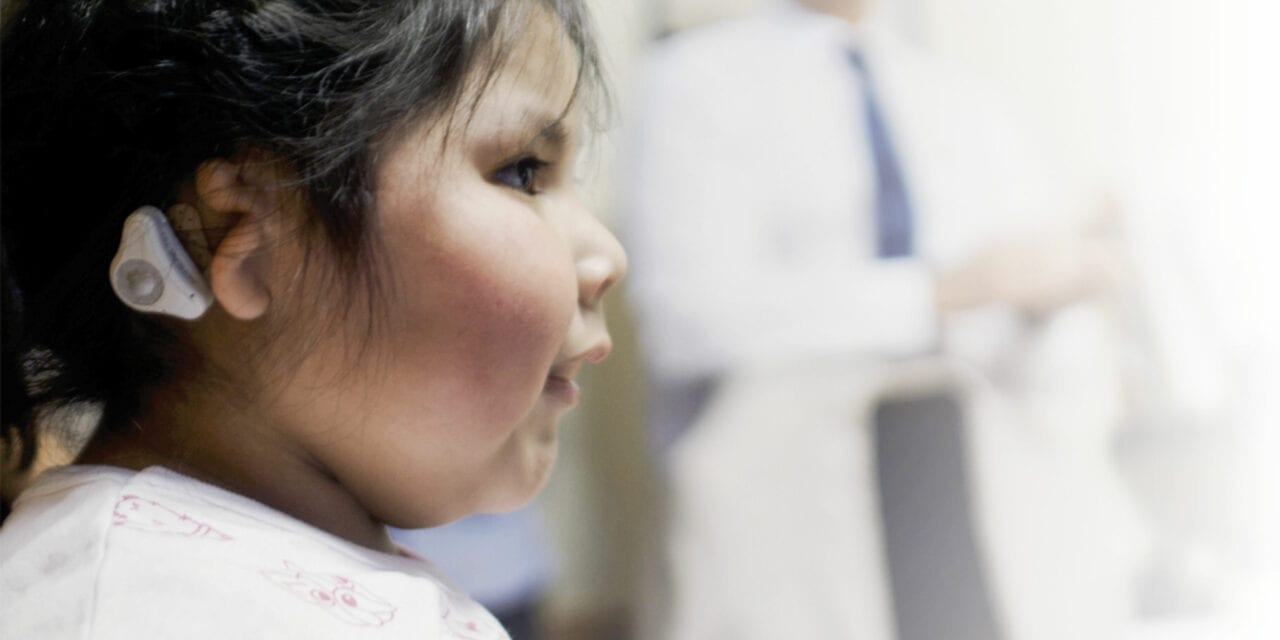
Children with cleft lip and/or cleft palate—the most common birth defect in the United States—present unique audiologic challenges that include a high incidence of otitis media with effusion (OME). Recently, the MED-EL ADHEAR system has opened up new options for these patients. Five case studies are presented here showing how this new system represents an innovative, non-surgical therapeutic option for children who are born with cleft palate.
Cleft lip and/or cleft palate occurs in one of every 575 live births, making it the most common major birth defect in the United States.1 A cleft occurs when structures improperly fuse during development to form an opening in the lip and/or palate. Most clefts are a result of genetic or environmental factors; however, other causes include chromosomal abnormalities or mechanically induced clefts. Clefts often exist as part of a larger craniofacial syndrome, such as Treacher Collins syndrome, Pierre Robin sequence, Apert Syndrome, Crouzon Syndrome, or Hemifacial microsomia.
Children born with a cleft or other craniofacial difference often require treatment provided by a coordinated team of healthcare providers who specialize in working with patients and families to address their specific and complex needs. Many such teams are approved by the American Cleft Palate-Craniofacial Association (ACPA, https://acpa-cpf.org).
Children with cleft lip and palate are at risk for speech and language disorders and are typically assessed by a speech-language pathologist on the cleft and craniofacial team. As an anatomical basis for chronic middle ear pathology exists, an audiologist should also ideally contribute to the care of the child. Abnormal function of the tensor and levator palatini muscles causes malfunction of the opening of the eustachian tube, which in turn causes poor ventilation and accumulation of fluids in the middle-ear space, resulting in a conductive hearing loss.
The occurrence of otitis media with effusion (OME) in children with cleft palate was first reported by Stool and Randall2 in 1967 and Paradise et al3 in 1969, with the incidence reportedly as high as 97%.4 Therefore, it is imperative to monitor hearing and identify hearing loss early in order to minimize the impact of abnormal or fluctuating hearing sensitivity on speech and language development.
Treating the middle-ear pathology of patients with cleft palate and craniofacial differences is challenging. Children with cleft palate often receive tympanostomy tubes at the time of cleft palate repair, if not before. Unfortunately, even after repair, chronic middle-ear pathology related to eustachian tube dysfunction persists in most (80%) patients.4 Children with cleft palate often require multiple sets of tubes which put them at increased risk for complications such as tympanosclerosis, cholesteatoma, and tympanic membrane perforation. Although the protocols for management of OME in the cleft palate population vary, research indicates that multiple tube placements in this population may not improve long-term hearing outcomes.5
Children presenting with chronic conductive hearing loss may require hearing technology to improve access to spoken language. However, traditional behind-the-ear amplification may not be an option due to fluctuating hearing sensitivity and otorrhea. The remaining options—preferential seating and use of an FM system—are often simply not enough to offset the potential negative impact of inconsistent auditory input.
In the presence of a conductive hearing loss, air conduction thresholds often fluctuate, while bone conduction thresholds typically remain stable and within normal limits. Given the underlying pathophysiology and clinical presentation, bone conduction systems offer a good solution to the problem of providing consistent access to auditory input. Unfortunately, advancements in bone conduction systems have focused on permanent conductive hearing loss or single-sided deafness with the goal of surgical implantation. A child with a cleft palate does not fit this model clinically and may also reject a softband for cosmetic reasons.
Recently, a new non-surgical bone conduction system became available in the United States which may offer an amplification solution for patients with cleft palate and/or chronic conductive hearing loss. This new option, the ADHEAR System by MED-EL has several advantages over previous pressure-based bone conduction options. The adhesive applicators allow for easy application and a more cosmetically appealing result as compared to other methods of retention. In addition, as a non-invasive, non-surgical means for improving hearing sensitivity, ADHEAR could potentially reduce the total number of otologic procedures resulting in better long-term hearing outcomes.
Given that most children with cleft palate have been shown to have OME by 4 months of age4 and continue to need treatment through age 10, the impact of this new system could dramatically improve speech/language development and academic outcomes.
Case Studies of Children with Hearing Impairment and Craniofacial Difference
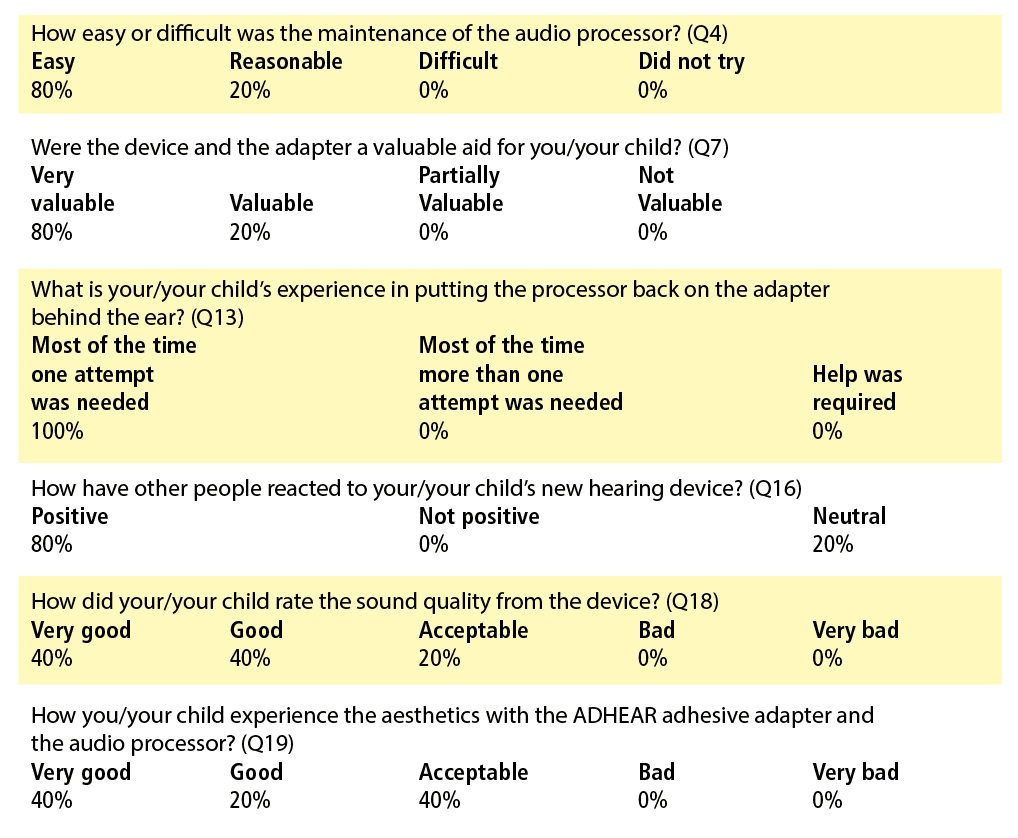
The following cases describe how ADHEAR was used for patients with cleft palate and other craniofacial differences in the Lancaster Cleft Palate Clinic, a major cleft craniofacial team approved by the ACPA. Audiometric testing was completed using a Natus Aurical Audiometer, and tympanometric testing was completed on a Maico MI44. In addition to aided and unaided testing, all families were asked to complete the Parent’s Evaluation of Aural/Oral Performance of Children (PEACH)6 and the ADHEAR Use and Satisfaction Questionnaire.7 Descriptions of degree of hearing loss follow the guidelines outlined by the American Speech-Language-Hearing Association (ASHA).8
Case 1
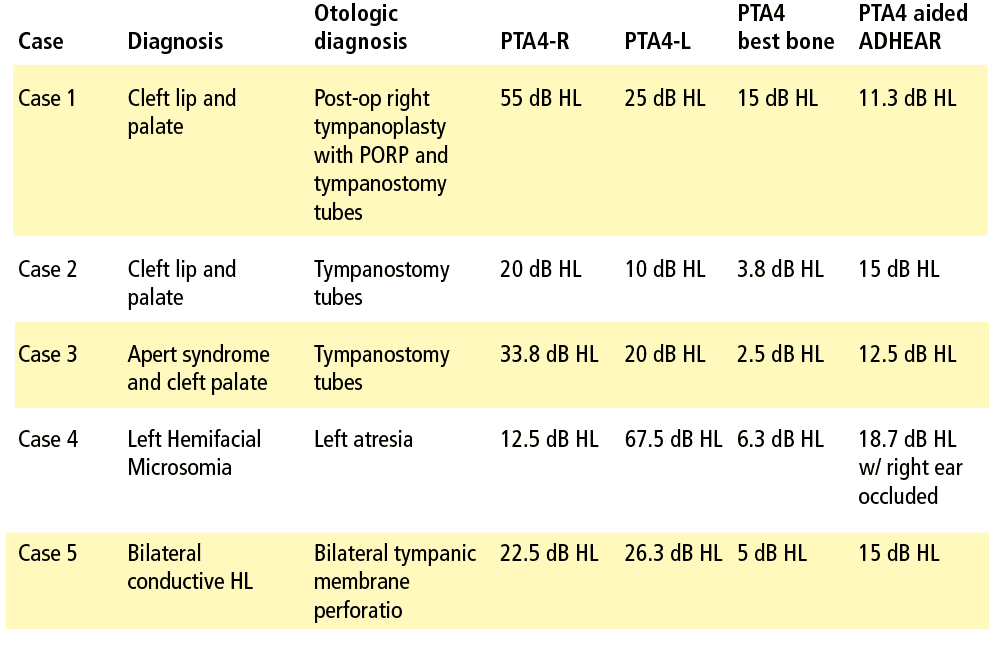
A 14-year-old female with a history of cleft lip and palate, eustachian tube dysfunction, and right conductive hearing loss was seen for audiometric evaluation following an unsuccessful right ossicular chain reconstruction. Post-operative audiologic testing revealed a moderately-severe rising to mild conductive hearing loss in the right ear and a mild low-frequency loss (250-500 Hz) rising to normal at 4000 Hz then sloping to a mild high-frequency conductive loss from 4000-8000 Hz.
The patient was provided surgical and amplification options. An in-clinic demonstration of ADHEAR was completed and the patient noted subjective improvement in hearing and ease of communication. She was fit with ADHEAR and was pleased with the comfort and sound quality. Aided sound field testing revealed thresholds in the normal to slight hearing loss range across frequencies.
She returned to the clinic 3 weeks post-fitting and with her father completed the questionnaires. She reported daily and consistent use, very good sound quality, acceptable aesthetics, and indicated that she was much more confident when wearing the processor because “I can hear so much better.” She reported, “I like listening to music now and I can talk on the phone using the Bluetooth adapter.” The patient’s father was pleased and felt that ADHEAR could bridge the gap until they were ready to reconsider surgical intervention to improve hearing.
Case 2
A 5-year-old male with non-syndromic cleft palate and bilateral tympanostomy tubes was seen in conjunction with the cleft/craniofacial team. Otoscopy revealed clear canals, tubes appeared open, and no active drainage was noted. Tympanometry revealed reduced compliance with large ear canal volumes consistent with open tubes bilaterally. Audiometric findings revealed slight low-frequency (250-500 Hz) hearing loss in the left ear and moderate to mild low-frequency (250-500 Hz) conductive hearing loss in the right ear. The patient’s mother reported frequent episodes of drainage from tubes accompanied by increased communicative difficulty and speech articulation errors.
An in-clinic demonstration of ADHEAR was completed and the patient’s mother decided to pursue a trial. Sound field aided results were obtained for speech and tones in the normal to slight hearing loss range from 250-4000 Hz.
At the follow-up appointment, the patient’s mother was interviewed and completed the questionnaires. According to parental report, there was daily and consistent use of ADHEAR regardless of active drainage. She noted improvement in ease of communication in the car and at the dinner table. Both had been difficult listening situations for the patient prior to fitting. She also reported a reduction in volume for the television and other electronic devices. Overall, the family was extremely pleased and decided to keep the device.
Case 3
A 15-year-old female with a history of cleft palate, Apert syndrome, chronic middle-ear pathology, and fluctuating hearing sensitivity was administered to by our cleft/craniofacial team. She had multiple sets of tympanostomy tubes and chronic ear drainage.
An in-clinic demonstration of ADHEAR was completed, and the patient’s mother expressed an interest in a trial period. She reported that her daughter’s fluctuating hearing sensitivity was becoming a barrier for social interactions with peers. Audiometric test findings revealed a mild low-frequency conductive loss in the left ear at 250-500 Hz and a slight to moderately severe conductive hearing loss in the right ear. She returned to the office for ADHEAR fitting, and aided thresholds were obtained in the normal to slight hearing loss range across the speech frequencies.
They returned to clinic 5 weeks after the fitting (follow-up was delayed due to surgery) and completed the questionnaires. Daily and consistent use of ADHEAR was reported. Both were pleased with the technology and noted improved ease of communication with family and peers. At a later follow-up appointment, the patient was experiencing otorrhea. Her mother reported prior to being fit with ADHEAR, the drainage would have resulted in frustration surrounding communicative difficulty. The family was pleased with the overall benefit but was especially grateful for the benefit during times of chronic drainage.
Case 4
A 4½-year-old female with left atresia and Hemifacial microsomia was referred for hearing aid consultation. Audiological findings indicated a complete unilateral conductive hearing loss on the left side with right-ear hearing sensitivity within normal limits. She had been followed regularly by our cleft/craniofacial team as well as a local otolaryngologist. The family was offered various amplification and surgical options but ultimately decided upon the ADHEAR System.
Aided sound field testing was completed with the better ear occluded. Aided thresholds were in the normal to slight hearing loss range with the exception of a mild loss noted at 4000 Hz. The questionnaires were completed at follow-up. The patient’s mother was extremely pleased with the ease of use and reported daily use of approximately 4-5 hours. She indicated that it was useful for off-side listening and that her daughter liked wearing the audio processor.
Case 5
A 9-year-old female with a history of cleft palate, eustachian tube dysfunction, multiple sets of tympanostomy tubes, and bilateral tympanic membrane perforations was seen as part of a cleft craniofacial team visit. The patient had moderate low-frequency conductive hearing loss rising to within normal limits bilaterally.
A demonstration of ADHEAR was completed after the patient’s mother expressed interest in amplification options while they were awaiting surgery. The patient returned to clinic for a hearing aid fitting. Aided sound field testing was completed and revealed good benefit from amplification with aided responses in the normal to slight hearing loss range.
Upon returning to the clinic after the hearing aid fitting, the patient’s mother reported improved ease of communication and noted that her daughter did not seem as tired or as irritable as she did before being fit with the ADHEAR. The patient’s mother also reported that her daughter could hear environmental noises like road noise when she was inside the home, which she had never noticed before.
Conclusion
In all cases presented, ADHEAR resulted in improved ease of communication and a high level of parental and patient satisfaction, as well as ease of use as evidenced by reports on the PEACH and the ADHEAR questionnaires.
Patients with cleft palate are not only at risk for speech language delays and auditory processing disorders,9 but also poorer academic outcomes and lower self-esteem as compared to children without clefts. Patients born with a cleft lip and palate undergo an average of 9-10 procedures by age 2110 and the number of tube insertions can be as high as 4 or more.5 The cost/benefit of repeated placement of tympanostomy tubes in this population is debatable, especially considering research that indicates that frequent use of tympanostomy tubes results in poorer long-term audiologic outcomes.5
ADHEAR offers a noninvasive, easy to use, cosmetically acceptable option which could provide consistent access to auditory stimuli for patients with cleft palate over the course of their treatment. There are no risks to overamplification and many benefits—providing a simple way to consistently access speech and environmental sounds.
Although the clinical cases described here represent a small sample, ADHEAR holds promise as an innovative, non-surgical therapeutic option for children who are born with cleft palate. The experiences above could be applied to other cases of chronic conductive hearing loss of uncertain duration.
Correspondence can be addressed to Dr Sharnetzka at: [email protected].
Citation for this article: Sharnetzka R. Hearing loss, cleft conditions, and craniofacial abnormalities. Hearing Review. 2020;27(10):14-17.
References
- American Cleft Palate-Craniofacial Association. Introduction to Cleft and Craniofacial Conditions. https://cleftline.org/family-resources/introduction-to-cleft-craniofacial-conditions/.
- Stool SE, Randall P. Unexpected ear disease in infants with cleft palate. Cleft Palate J. 1967;4(2):99-103.
- Paradise JL, Bluestone CD, Felder H. The universality of otitis media in 50 infants with cleft palate. Pediatrics. 1969;44(1):35-42.
- Dhillon RS. The middle ear in cleft palate children pre and post palatal closure. J Royal Soc Med. 1988;81:710-713.
- Kim E, Kanack MD, Dang-Vu MD, Carvalho D, Jones MC, Gosman AA. Evaluation of ventilation tube placement and long-term audiologic outcome in children with cleft palate. Cleft Palate-Craniofacial J. 2017;54(6):650-655.
- Ching TYC, Hill M. The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) scale: Normative data. J Am Acad Aud. 2007;18:220-235.
- ADHEAR Use and Satisfaction Questionnaire, MED-EL Corporation. Rev 1.0 5/2019.
- American Speech-Language-Hearing Association (ASHA). Degree of Hearing Loss. https://www.asha.org/public/hearing/Degree-of-Hearing-Loss/.
- Ramos do Amaral MI, Martins JE, Colella dos Santos MF. A study on the hearing of children with non-syndromic cleft palate/lip. Braz J Otorhin. 2009;76(2):164-171.
- MacIntyre JK, Sethi H, Schonbrunner A, Proudfoot J, Jones M, Gosman A. Number of surgical procedures for patients with cleft lip and palate from birth to 21 years old at a single children’s hospital. Ann Plas Surg. 2016;76:S205-S208.

No comments:
Post a Comment
We welcome and encourage all readers to post feedback, however, we reserve the right to remove any comments that are deemed offensive or unrelated to the topic of discussion. Thank you for understanding and for helping us to foster a healthy environment for the families that we serve.